Alkenes are essential to many chemical and industrial processes, where they serve as fundamental building blocks of many products. Known for their reactive carbon-carbon double bonds (C=C) in their structure, alkenes often form key intermediates during chemical reactions. These key intermediates play a crucial role in transforming reactants into final products, for example, they facilitate the synthesis of polyethylene, a common packaging plastic. In fact, alkenes are integral to the synthesis of detergents, cosmetics, pharmaceuticals, lubricants, and industrial solvents, highlighting their importance and value in modern manufacturing processes.
Alkenes are unsaturated hydrocarbons that contain carbon-carbon double bonds (C=C) somewhere in their structure. Currently, there are several methods for producing alkenes and their intermediates, including steam cracking, dehydrogenation, elimination reactions, and the Wittig reaction. However, each method comes with its own limitations. Steam cracking and dehydrogenation require high temperatures, and are therefore both energy-intensive and expensive. Elimination reactions involve strong bases, which can pose safety risks and lead to unwanted side reactions. The Wittig reaction on the other hand generates phosphine oxide waste, which is challenging to recycle.
Furthermore, each of these well-established alkene intermediate synthesis methods requires unique starting materials. For example, the Wittig reaction is effective in converting ketones to alkenes, whereas the elimination reaction can only produce alkenes from halides.
Seeking to streamline alkene intermediate synthesis, and establish a more efficient and sustainable production method, researchers at NUS sought to define a series of reactions that could produce alkene and their intermediates from different substrates with distinct chemical properties.
Breakthrough in alkene intermediate synthesis
Led by Associate Professor Wu Jie from NUS Chemistry, the team of researchers successfully developed a universal one-pot photochemical strategy that can produce alkenes and their intermediates from a variety of starting materials, including carboxylic acids, alcohols and alkanes.
Their method draws inspiration from the Norrish Type II photochemical reaction, which uses light to convert carbonyl compounds into alkenes. To mitigate the chance of competing reactions reducing the yield of the desired alkene, the team added a ketone group to each starting material, thereby allowing them to control which reactions would occur.
Published in
Nature Chemistry, their method was shown to simplify existing procedures and in turn, reduce time, save resources, and reduce waste.
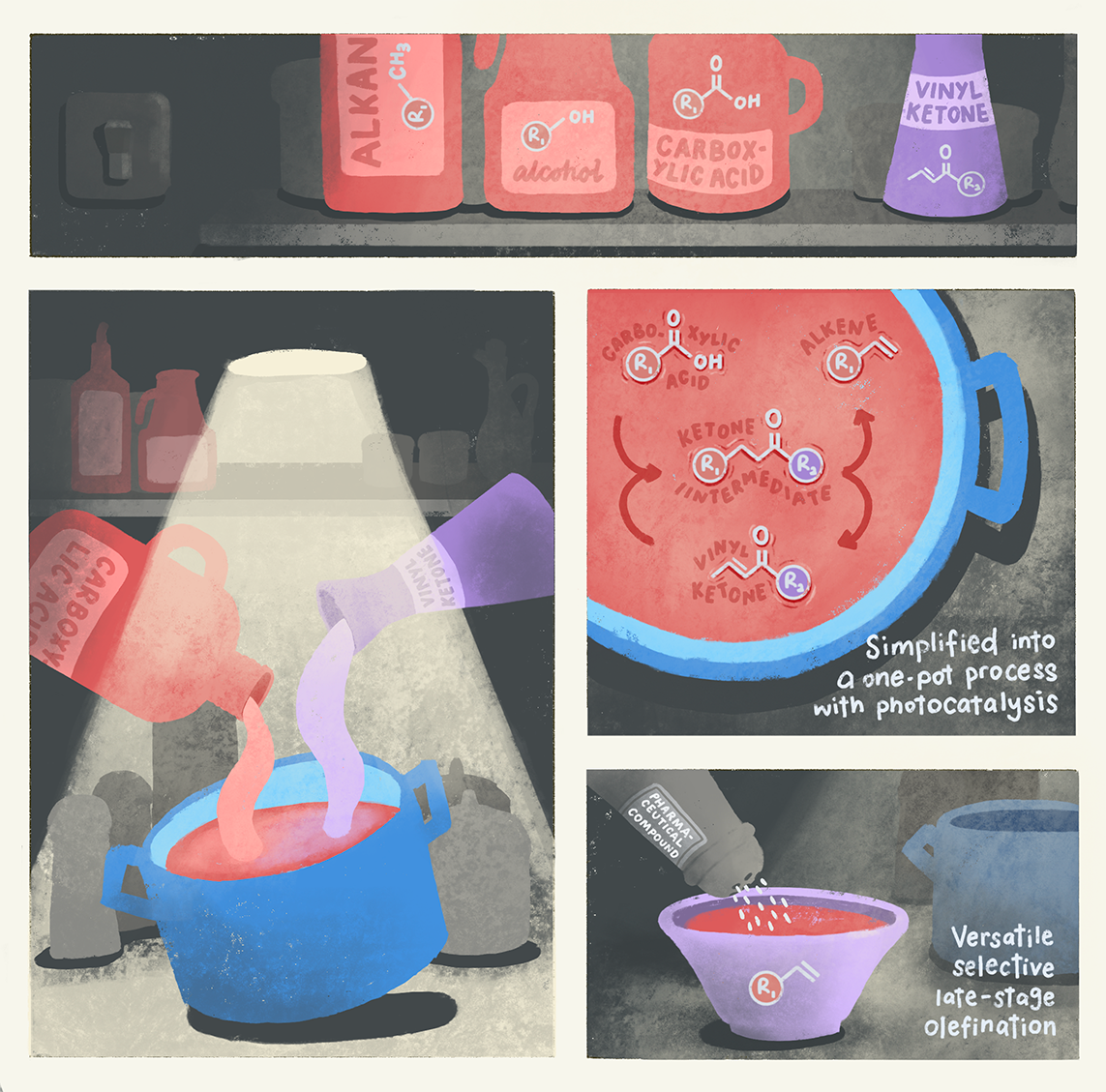
This integrated photochemical method means alkenes can be produced directly in a one-pot process from common and inexpensive starting materials such as carboxylic acids, alcohols, and alkanes, which would otherwise require multiple steps and expensive reagents.
Showing the efficacy of their reactions, the team produced alkene intermediates from 5-cholenic acid, a common carboxylic acid that can be converted into bioactive compounds, including DAF-12 antagonists, to study hormone signalling and development in biological research.
While the existing method to create these alkene intermediates involves a nine-step process, the new approach allows the process to be simplified into two steps. In this case, 5-cholenic acid is directly converted into the corresponding alkene via the photochemical method, after which it undergoes a difluoromethylation reaction to produce the alkene intermediate. This showcases the potential for this novel method to drastically simplify complex molecule synthesis.
“We have successfully integrated our strategy into the construction of complex bioactive molecules and natural products to streamline previous synthetic routes. Given that alkenes frequently act as intermediates in complex molecular architectures, our method shows significant promise for simplifying the synthesis of useful compounds,” Assoc Prof Wu added.
Having established a method to convert carboxylic acids into alkenes, the team was also able to synthesise alkenes from other starting materials including alcohols and alkanes, thereby opening new possibilities for synthetic chemistry.
Our strategy greatly expands the range of raw materials for synthesising alkenes, even though the chemical reactivities of these raw materials are different. It allows us to make certain alkenes that were previously difficult or even impossible to synthesise with traditional methods.
The new method also allows for late-stage on-demand modification of molecules, especially in the pharmaceutical industry, where the flexibility to modify a drug’s molecular structure without starting from scratch allows for more efficient use of resources and reduces waste.
“Our method allows us to directly add an alkene group to natural products and pharmaceutical compounds. This means we can make targeted adjustments to enhance the drug’s properties or effectiveness in a more efficient way,” Assoc Prof Wu explained.
By using photocatalysis methods on readily available and inexpensive starting materials such as carboxylic acids, alcohols, and alkanes, the new one-pot approach promises to transform manufacturing processes in the chemical industry with a streamlined, greener, more energy-efficient alternative to existing methods of alkene synthesis.
References
Zeng, H., Yin, R., Zhao, Y., Ma, J. A., & Wu, J. (2024). Modular alkene synthesis from carboxylic acids, alcohols and alkanes via integrated photocatalysis. Nature Chemistry, 1-9.

